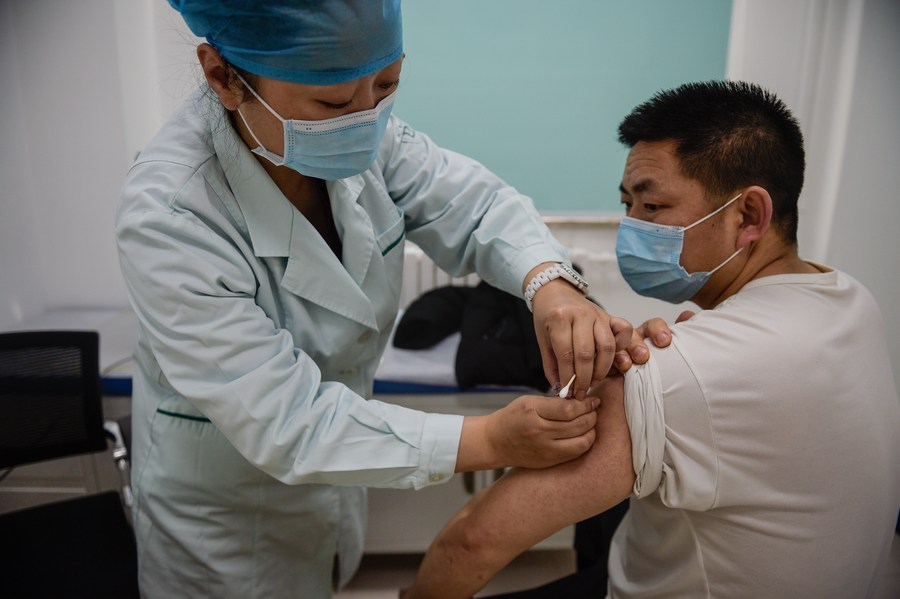
COVID-19 vaccines, litigation-shield laws go hand in hand

Shakespeare's body of work is filled with remarkable characters. In Henry VI (Part 2), from 1591, Dick the Butcher says "Let's kill all the lawyers". This is regularly read as a reflection on how lawyers, over 400 years ago, were already renowned for twisting words and events to the advantage of clients and to their own advantage. Lawyers, it is worth remembering, have not historically been required, like doctors, to swear first, to do no harm upon initiation to their profession.
In 1986, the US established the National Vaccine Injury Compensation Program (NVICP) in response to a threat to vaccine supplies arising out of multiple, big-dollar lawsuits claiming damages for side effects from certain vaccines administered in the 1980s. American Public Health officials said the claimed side effects were regularly ill-founded but juries kept finding in favor of plaintiffs.
Nowhere in the developed world is civil litigation more prevalent than in the US. The legislation establishing the NVICP stopped almost all vaccine injury lawsuits, however, by effectively banning them. Instead, anyone claiming to have been harmed through vaccination had first to sue to recover from the NVICP itself by establishing an injury linkage in accord with the rules set down in that program. Time limits to sue applied and limits on awards were stipulated. Fault did not need to be shown — but a clear link between vaccine and injury had to be established. Funding was provided via a small excise tax (75 cents) on every purchased dose of a vaccine covered under the NVICP.
The NVICP tracked the introduction, in the 1960s, of German and French public compensation schemes for vaccine injuries, which also sidelined private actions.
The NVICP was a radical move for the US. But the alternative was either a drying up of vaccine supplies (and research) or a potentially huge increase in vaccine costs to cover possible legal claims. Both outcomes were seen as contrary to the broad public interest. Vaccine development and production were put back on track.
Somewhat controversial, additional protection for US vaccine makers was provided in 2005 (when Avian Flu concerns were high) with the Public Readiness and Preparedness Act.
Amongst other things, it covered new vaccine development and production — and emergency use — once a public health emergency had been declared.
This powerful litigation shield, enacted by Congress, has been a significant factor in allowing Pfizer-BioNTech and Moderna, to develop and deploy, with exceptional speed, two anti-COVID vaccines in the US using a new vaccine technique.
Numerous other jurisdictions have enacted similar laws to shield vaccine makers from adamant lawyering, in order to protect the public interest.
Vaccines can never be guaranteed to be absolutely free of side effects. When administered to any large population there will be a very small number who may be particularly vulnerable to side effects of varying seriousness, due to personal physiology and medical history. This risk rises with emergency vaccine usage. The compensation schemes outlined above are designed to look after such persons, while closing the door on proliferating litigation.
In addition to relying on a legislative shield, vaccine makers can also protect themselves by contract. It is not possible to do this on an individual basis with each person being immunized. But it is common — as we are seeing with COVID vaccines — for governments to be the primary purchaser of vaccine supplies and it is also unexceptional for governments to contract that they will not sue in relation to certain specified matters associated with vaccine production and supply. This helps speed delivery — and it also lowers costs.
We need to remember that vaccine creation and production is intensely complex and demanding. The reputation concerns of manufacturers provide crucial, initial protection against the risk of scientific recklessness. More importantly, vaccines are subject to exacting public verification protocols prior to being authorized for public use.
Can we be sure nothing can go wrong, especially with vaccines produced during a huge, ongoing public health emergency? No, we cannot. But if we delay continuously to try and eliminate all possible risks, will many more die and still more suffer? Yes, they will.
Bearing in mind both the need-pressures and safeguards outlined above, many jurisdictions have agreed that certain newly created, widely tested COVID vaccines should be granted emergency use status to avoid the delay involved in waiting until such vaccines becoming fully registered.
Hong Kong has sensibly taken this approach. We are fortunate, too, that the Government has now secured enough vaccine doses from three sources (Sinovac, AstraZeneca and Fosun-Pfizer-BioNTech) to inoculate the entire HKSAR population.
The COVID pandemic has disrupted life across the planet to an extraordinary degree. The consequences have been devastating at many levels and, regularly lethal. One primary positive story to emerge from this immense misfortune has been the development, at record speed, of a number of promising vaccines. These offer the clearest chance of laying foundations for a return to long-term, public health normality. Moreover, this experience signals what may be possible as further pandemics arise. There are now some inspiring, fresh pathways to follow.
We did not have to kill all the lawyers to secure this outcome — nor would we want to. Shakespeare would surely agree, however, that it is fortunate smart law-making beginning decades ago has ensured that predatory lawyering could not defer — or stop — urgently needed vaccine research aimed at controlling the worst public health crisis in over 100 years.
The author is a visiting professor at the law faculty of the University of Hong Kong.
The views do not necessarily reflect those of China Daily.